

Updated: 2024-01-19

Recently, a paper titled "Dealuminated Beta zeolite reverses Ostwald ripening for durable copper nanoparticle catalysts," led by Professor Xiao Fengshou and Researcher Liang Wang from the School of Chemical and Biological Engineering at Zhejiang University, and in collaboration with Professor Jiabi Ma of the School of Chemistry and Chemical Engineering at the Beijing Institute of Technology (BIT), and researchers of other institutes, was published in the journal Science.
Catalysts based on copper nanoparticles have been widely used in industry, but in a chemical atmosphere, the nanoparticles tend to sinter into larger ones, thereby reducing the catalyst's performance. The paper discusses the use of dealuminated Beta zeolite to load Cu nanoparticles (Cu/Beta-deAl). It was found that these particles reduced in size from ~5.6 nm to ~2.4 nm in methanol vapor at 200°C, which is contrary to the typical sintering phenomenon.
Additionally, a reverse Ostwald ripening process was discovered, where methanol-activated migratory copper sites were captured using silanol nests, and the copper in the nests served as new nucleation sites for forming small nanoparticles. This unique feature, distinct from conventional sintering pathways, led to the preparation of stable catalysts for the hydrogenation of dimethyl oxalate (DMO) in the industry.
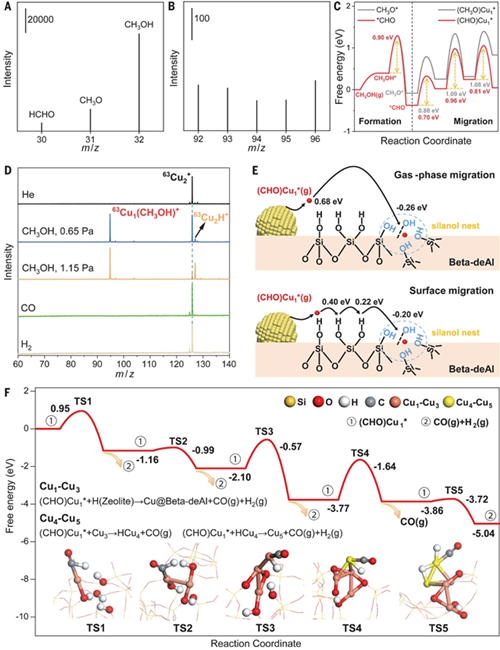
Figure 1: Mass spectrometry experiments and mechanistic analysis of the reverse maturation process.
In this paper, the BIT played a collaborative role and was responsible for the technical support of in-situ structural studies on copper particle catalysts. The study utilized a self-designed in-situ reaction device with time-of-flight mass spectrometry as the main tool, using well-defined, controllable, and highly reproducible gas-phase clusters as research models for studying the interaction between 63Cu2+ cations and small gas molecules such as methanol, H2, and CO. The cluster study results indicated that only methanol molecules could cause Cu–Cu bond breaking, while the other two gases showed inertness. This provided crucial information for capturing key intermediates in the catalytic process.
The first author affiliation of the paper is the School of Chemical and Biological Engineering at Zhejiang University. Professor Xiaofeng Shou and researcher Liang Wang from Zhejiang University, Professor Xiaoming Cao from the East China University of Science and Technology, and Professor Jiabi Ma from BIT are the corresponding authors of the paper, and doctoral student Wang Ming from the research group participated in the work.
Paper Link: https://www.science.org/doi/10.1126/science.adj1962
About the research group:
Jiabi Ma is a professor and Ph.D. supervisor. He has made a series of achievements in the activation and transformation of stable molecules such as transition metal nitrogen and oxide cluster structures, as well as N2, CO2, and hydrocarbons. So far, Ma has published more than 60 articles in journals such as Science, J. Am. Chem. Soc., and Angew. Chem. Int. Ed, etc. He has led projects such as the National Youth Talent Program, the National Key R&D Program, and the Beijing Natural Science Foundation. He has also been invited to serve as a young editorial board member of Chin. Chem. Lett.














